
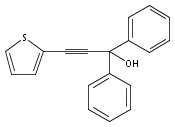
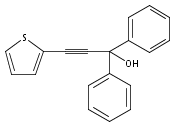
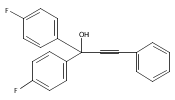
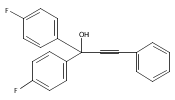
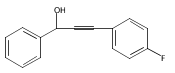
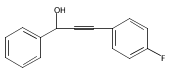
The acceleration effect from the addition of N-fluorobenzenesulfonimide (NFSI) to a samarium(III) triflate catalyzed Meyer–Schuster rearrangement under microwave irradiation was studied. Reaction rate plots indicate that the Sm(OTf)3/NFSI system is especially suitable for unreactive substrates towards the Meyer–Schuster rearrangement. The mechanism was probed by a crossover experiment using 18O-labeled substrates.



![2-(3-HYDROXY-4-METHOXYPHENYL)ETHYL 3-O-(6-DEOXY-WEI -L-MANNOPYRANOSYL)-6-O-[(2E)-3-(4-HYDROXY-3-METHOXYPHENYL)-2-PROPENOYL]-WEI -D-GLUCOPYRANOSIDE](http://img.cochemist.com/ccimg/1204400/1204313-51-8.png)
![2-(3-HYDROXY-4-METHOXYPHENYL)ETHYL 3-O-(6-DEOXY-WEI -L-MANNOPYRANOSYL)-6-O-[(2E)-3-(4-HYDROXY-3-METHOXYPHENYL)-2-PROPENOYL]-WEI -D-GLUCOPYRANOSIDE](http://img.cochemist.com/ccimg/1204400/1204313-51-8_b.png)


![2-PROPENAMIDE, 3-CHLORO-N-PHENYL-N-[1-(PHENYLMETHYL)-4-PIPERIDINYL]-](http://img.cochemist.com/ccimg/851300/851223-93-3.png)
![2-PROPENAMIDE, 3-CHLORO-N-PHENYL-N-[1-(PHENYLMETHYL)-4-PIPERIDINYL]-](http://img.cochemist.com/ccimg/851300/851223-93-3_b.png)
![Benzenamine, 2-[(2-methoxyphenyl)methoxy]-](http://img.cochemist.com/ccimg/844900/844897-55-8.png)
![Benzenamine, 2-[(2-methoxyphenyl)methoxy]-](http://img.cochemist.com/ccimg/844900/844897-55-8_b.png)
![THIOPHENE, 2-ISOCYANATO-5-[2-(TRIFLUOROMETHYL)PHENYL]-](http://img.cochemist.com/ccimg/844900/844897-54-7.png)
![THIOPHENE, 2-ISOCYANATO-5-[2-(TRIFLUOROMETHYL)PHENYL]-](http://img.cochemist.com/ccimg/844900/844897-54-7_b.png)

