Co-reporter:U. B. Rao Khandavilli;Balakrishna R. Bhogala;Anita R. Maguire
Chemical Communications 2017 vol. 53(Issue 23) pp:3381-3384
Publication Date(Web):2017/03/16
DOI:10.1039/C7CC01091E
The mechanical flexibility known in the antihyperuricemia drug probenecid has been extended into multi-component systems using co-formers with two donor or acceptor sites, in contrast to systems with a single H-bond acceptor that exhibit brittle behaviour. The piperazinium salt demonstrates that GRAS co-formers can be used to maintain mechanical flexibility with drug molecules.
Co-reporter:Jerome G. P. Wicker;Lorraine M. Crowley;Oliver Robshaw;Edmund J. Little;Stephen P. Stokes;Richard I. Cooper
CrystEngComm (1999-Present) 2017 vol. 19(Issue 36) pp:5336-5340
Publication Date(Web):2017/09/18
DOI:10.1039/C7CE00587C
A data-driven approach to predicting co-crystal formation reduces the number of experiments required to successfully produce new co-crystals. A machine learning algorithm trained on an in-house set of co-crystallization experiments results in a 2.6-fold enrichment of successful co-crystal formation in a ranked list of co-formers, using an unseen set of paracetamol test experiments.
Co-reporter:U. B. Rao Khandavilli, Matteo Lusi, Balakrishna R. Bhogala, Anita R. Maguire, Matthias Stein and Simon E. Lawrence
Chemical Communications 2016 vol. 52(Issue 53) pp:8309-8312
Publication Date(Web):08 Jun 2016
DOI:10.1039/C6CC04148E
The crystal structure containing (±)-3-methyl-2-phenylbutyramide with salicylic acid is the first example of a kryptoracemate co-crystal. It exhibits the first temperature mediated reversible single-crystal to single-crystal transition between two kryptoracemate forms, in addition to crystallising in another, racemic, form. Theoretical calculations and structural analysis reveal that there are only small differences in both energy and packing arrangements between the three forms. These results suggest that co-crystals can be an opportunity to investigate kryptoracemate behaviour.
Co-reporter:A. S. Sinha, U. B. Rao Khandavilli, E. L. O’Connor, B. J. Deadman, A. R. Maguire and S. E. Lawrence
CrystEngComm 2015 vol. 17(Issue 26) pp:4832-4841
Publication Date(Web):29 May 2015
DOI:10.1039/C5CE00777A
Sinapic acid (SA) is a nutraceutical with known anti-oxidant, anti-microbial, anti-inflammatory, anti-cancer, and anti-anxiety properties. Novel co-crystals of SA were prepared with co-formers belonging to the category of GRAS [isonicotinic acid (INC), nicotinamide (NIA)], non-GRAS [4-pyridinecarbonitrile (PYC)], and active pharmaceutical ingredients (APIs) [6-propyl-2-thiouracil (PTU)] list of compounds. Structural study based on the X-ray crystal structures revealed the intermolecular hydrogen-bonded interactions and molecular packing. The crystal structure of sinapic acid shows the anticipated acid–acid homodimer along with discrete hydrogen bonds between the acid carbonyl and the phenolic moiety. The robust acid–acid homodimer appears to be very stable and is retained in the structures of two co-crystals (SA·NIA and SA·PYC). In these cases, co-crystallization occurs via intermolecular phenol O–H⋯Naromatic hydrogen bonds between the co-formers. In the SA·PTU·2MeCN co-crystal the acid–acid homodimer gives way to the anticipated acid–amide heterodimer, with the phenolic moiety of SA hydrogen-bonded to acetonitrile. Attempts at obtaining the desolvated co-crystal led to lattice breakdown, thus highlighting the importance of acetonitrile in the formation of the co-crystal. Among the co-crystals examined, SA·INC (5 weeks), SA·NIA (8 weeks) and SA·PYC (5 weeks) were found to be stable under accelerated humidity conditions (40 °C, 75% RH), whereas SA·PTU·2MeCN decomposed after one week into individual components due to solvent loss.
Co-reporter:Abhijeet S. Sinha, Anita R. Maguire, and Simon E. Lawrence
Crystal Growth & Design 2015 Volume 15(Issue 2) pp:984-1009
Publication Date(Web):December 19, 2014
DOI:10.1021/cg501009c
Cocrystallization has emerged over the past decade as an attractive technique for modification of the physicochemical properties of compounds used as active pharmaceutical ingredients (APIs), complementing more traditional methods such as salt formation. Nutraceuticals, with associated health benefits and/or medicinal properties, are attractive as coformers due to their ready availability, known pharmacological profile, and natural origin, in addition to offering a dual therapy approach. Successful studies of favorably altering the physicochemical properties of APIs through cocrystallization with nutraceuticals are highlighted in this review. Many of the key functional groups commonly seen in nutraceuticals (e.g., acids, phenols) underpin robust supramolecular synthons in crystal engineering. This review assesses the structural data available to date across a diverse range of nutraceuticals, both in pure form and in multicomponent materials, and identifies the persistent supramolecular features present. This insight will ultimately enable predictive and controlled assembly of functional materials incorporating nutraceuticals together with APIs.
Co-reporter:Kevin S. Eccles, Robin E. Morrison, Abhijeet S. Sinha, Anita R. Maguire, and Simon E. Lawrence
Crystal Growth & Design 2015 Volume 15(Issue 7) pp:3442
Publication Date(Web):June 5, 2015
DOI:10.1021/acs.cgd.5b00513
Cocrystallization utilizing halogen bonding involving the thiocarbonyl functional group of a series of primary aromatic thioamides has been investigated. The well-known organoiodide 1,4-diiodotetrafluorobenzene was utilized as the halogen bond donor and the C═S···I halogen bond was established as a robust supramolecular synthon in these systems. Weak N–H···S hydrogen bonding involving the thioamides influences the overall supramolecular architectures, meaning that there is a diverse range of structural motifs and cocrystal stoichiometries observed. The majority (60%) of the cocrystals obtained have a 2:1 ratio of thioamide/organiodide with the latter present over an inversion center. The higher ratio of organoiodide seen in the other cocrystals is achieved by additional I···I and I···π halogen bonding. The C═S···I halogen bond is replaced by N···I halogen bonding in the one cocrystal containing a pyridyl-substituted thioamide. The ability of the thioamides to form cocrystals and the strength of the halogen bond were influenced by the nature of the substituents on the aromatic ring, with derivatives containing electron donating groups most likely to form cocrystals. Calculated molecular electrostatic potential values on the sulfur atom in the thioamides corroborate these experimental results.
Co-reporter:U. B. Rao Khandavilli, Balakrishna R. Bhogala, Anita R. Maguire and Simon E. Lawrence
Chemical Communications 2017 - vol. 53(Issue 23) pp:NaN3384-3384
Publication Date(Web):2017/03/03
DOI:10.1039/C7CC01091E
The mechanical flexibility known in the antihyperuricemia drug probenecid has been extended into multi-component systems using co-formers with two donor or acceptor sites, in contrast to systems with a single H-bond acceptor that exhibit brittle behaviour. The piperazinium salt demonstrates that GRAS co-formers can be used to maintain mechanical flexibility with drug molecules.
Co-reporter:U. B. Rao Khandavilli, Matteo Lusi, Balakrishna R. Bhogala, Anita R. Maguire, Matthias Stein and Simon E. Lawrence
Chemical Communications 2016 - vol. 52(Issue 53) pp:NaN8312-8312
Publication Date(Web):2016/06/08
DOI:10.1039/C6CC04148E
The crystal structure containing (±)-3-methyl-2-phenylbutyramide with salicylic acid is the first example of a kryptoracemate co-crystal. It exhibits the first temperature mediated reversible single-crystal to single-crystal transition between two kryptoracemate forms, in addition to crystallising in another, racemic, form. Theoretical calculations and structural analysis reveal that there are only small differences in both energy and packing arrangements between the three forms. These results suggest that co-crystals can be an opportunity to investigate kryptoracemate behaviour.


![Benzene, [(octylthio)methyl]-](http://img.cochemist.com/ccimg/85300/85293-39-6.png)
![Benzene, [(octylthio)methyl]-](http://img.cochemist.com/ccimg/85300/85293-39-6_b.png)
![Benzene, 1-methyl-4-[[(4-methylphenyl)methyl]sulfinyl]-](http://img.cochemist.com/ccimg/95200/95126-91-3.png)
![Benzene, 1-methyl-4-[[(4-methylphenyl)methyl]sulfinyl]-](http://img.cochemist.com/ccimg/95200/95126-91-3_b.png)
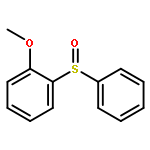
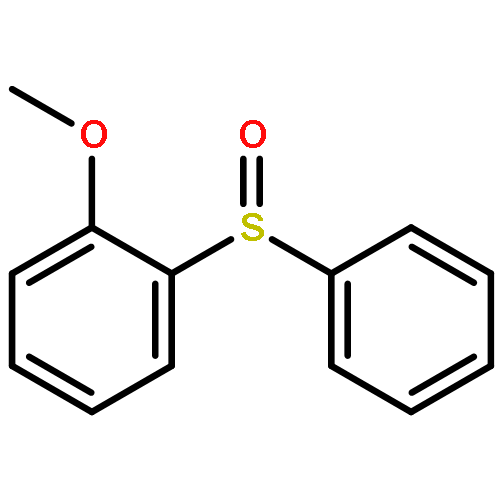
![Benzene, 1-fluoro-4-[[(4-fluorophenyl)methyl]thio]-](http://img.cochemist.com/ccimg/900/836-45-3.png)
![Benzene, 1-fluoro-4-[[(4-fluorophenyl)methyl]thio]-](http://img.cochemist.com/ccimg/900/836-45-3_b.png)
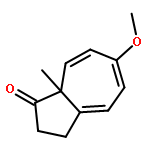
![Benzene, 1-methoxy-3-[(phenylmethyl)thio]-](http://img.cochemist.com/ccimg/695200/695160-62-4.png)
![Benzene, 1-methoxy-3-[(phenylmethyl)thio]-](http://img.cochemist.com/ccimg/695200/695160-62-4_b.png)
![Benzene, 1-methoxy-2-[[(4-methylphenyl)thio]methyl]-](http://img.cochemist.com/ccimg/138300/138298-13-2.png)
![Benzene, 1-methoxy-2-[[(4-methylphenyl)thio]methyl]-](http://img.cochemist.com/ccimg/138300/138298-13-2_b.png)