Co-reporter: Shaowei Zhang, Han Li, Eryue Duan, Zongsu Han, Leilei Li, Jinkui Tang, Wei Shi, and Peng Cheng
pp: 1202-1207
Publication Date(Web):January 11, 2016
DOI: 10.1021/acs.inorgchem.5b02378
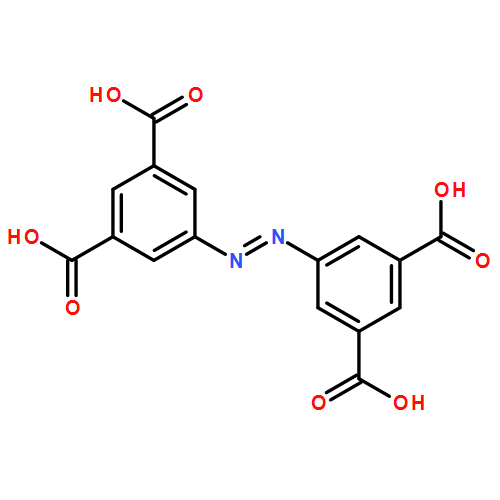
The solvothermal reaction of DyCl3·6H2O, Ni(NO3)2·6H2O, and H4abtc ligands (H4abtc = 3,3′,5,5′-azobenzene-tetracarboxylic acid) in the mixed DMF/H2O solvents (DMF = N,N-dimethylformamide) produced a three-dimensional (3D) NiII–DyIII heterometallic coordination polymer (HCP) formulated as {[NH2(CH3)2]2[NiDy2(HCOO)2(abtc)2]}n (1). In 1, DyIII and NiII ions interconnect through carboxylic O donors of abtc4– ligands to generate a linear trimer “Hourglass”-type {NiDy2} cluster, and the adjacent trinuclear {NiDy2} units are bridged by HCOO– groups to give a 1D “ladder” chain, which is further bridged by abtc4– ligands to form a new topology and named as “zsw3”. Alternating-current magnetic susceptibility results indicate that 1 exhibits frequency-dependent out-of-phase signals with two relaxation processes, which suggests that it shows single-molecule magnet (SMM) behavior and represents the first example by using an SMM cluster as the building block to create a 3D Ni–Ln HCP, to the best of our knowledge. The energy barriers for 1 under a 1000 Oe applied direct current magnetic field are estimated from Arrhenius plots to be 40 and 42 K at higher and lower frequencies, respectively. Additionally, the crystalline structure of 1 could be stable to at least 310 °C, supported by thermogravimetric analyses and in situ variable-temperature powder X-ray diffraction patterns.