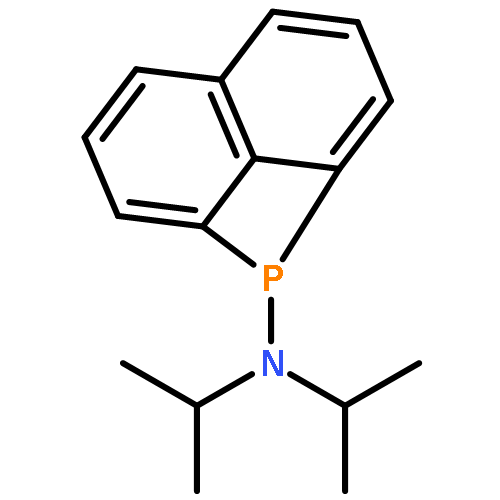
A synthetic route was developed for a novel bicyclic diphosphine 5 in which two structurally robust 1,8-naphthalenediyl groups clamp the P–P bond of the diphosphine to form a butterfly structure. The bicyclic framework was synthesized by a ring-opening dimerization of a strained four-membered phosphacycle of iPr2NP-peri-bridged naphthalene 8. A toluene solution of 8 was heated for 2 days in the presence of O2 and one equivalent of H2O to give a mixture of oxides of the target diphosphine 5, then the mixture of oxides was reduced with HSiCl3 to afford 5 in a moderate yield. The crystal structure of 5 confirmed that the P–P bond and the two naphthalene groups form a butterfly molecular structure, and the two lone pairs on the respective phosphorus centers are disposed in a synperiplanar configuration. These two phosphorus donor atoms bind two AuCl fragments to form a unique “U-shape” binuclear complex (μ-5)-[AuCl]2 in which the two Au(I) centers are separated by ca. 4.4 Å that indicates the negligible intramolecular Au–Au interaction. In the crystal, the two binuclear complexes interlocked with each other through multiple intermolecular Au–Au interactions between the two U-shape units.Dimerization reaction of phosphorus-atom peri-bridged naphthalene having a strained four-membered heterocycle gave bis(1,8-naphthalenediyl)diphosphine, in which the two lone pairs on the respective phosphorus centers are disposed in a synperiplanar configuration.
![Image for unlabelled figure]()