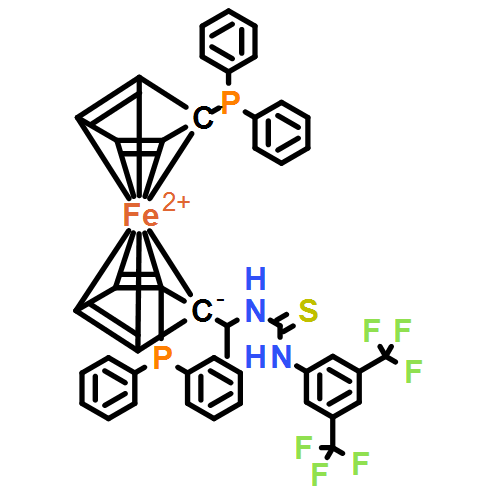
Abstract
Asymmetric hydrogenation of unprotected NH imines catalyzed by rhodium/bis(phosphine)-thiourea provided chiral amines with up to 97 % yield and 95 % ee. 1H NMR studies, coupled with control experiments, implied that catalytic chloride-bound intermediates were involved in the mechanism through a dual hydrogen-bonding interaction. Deuteration experiments proved that the hydrogenation proceeded through a pathway consistent with an imine.