Abstract
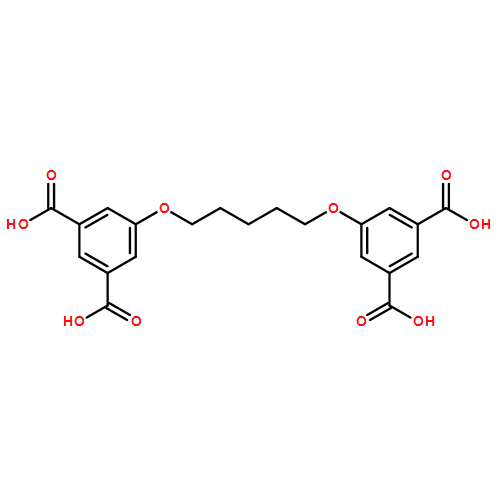
In our continuing quest to develop a metal–organic framework (MOF)-catalyzed tandem pyrrole acylation–Nazarov cyclization reaction with α,β-unsaturated carboxylic acids for the synthesis of cyclopentenone[b]pyrroles, which are key intermediates in the synthesis of natural product (±)-roseophilin, a series of template-induced Zn-based (1–3) metal-organic frameworks (MOFs) have been solvothermally synthesized and characterized. Structural conversions from non-porous MOF 1 to porous MOF 2, and back to non-porous MOF 3 arising from the different concentrations of template guest have been observed. The anion–π interactions between the template guests and ligands could affect the configuration of ligands and further tailor the frameworks of 1–3. Futhermore, MOFs 1–3 have shown to be effective heterogeneous catalysts for the tandem acylation–Nazarov cyclization reaction. In particular, the unique structural features of 2, including accessible catalytic sites and suitable channel size and shape, endow 2 with all of the desired features for the MOF-catalyzed tandem acylation–Nazarov cyclization reaction, including heterogeneous catalyst, high catalytic activity, robustness, and excellent selectivity. A plausible mechanism for the catalytic reaction has been proposed and the structure–reactivity relationship has been further clarified. Making use of 2 as a heterogeneous catalyst for the reaction could greatly increase the yield of total synthesis of (±)-roseophilin.