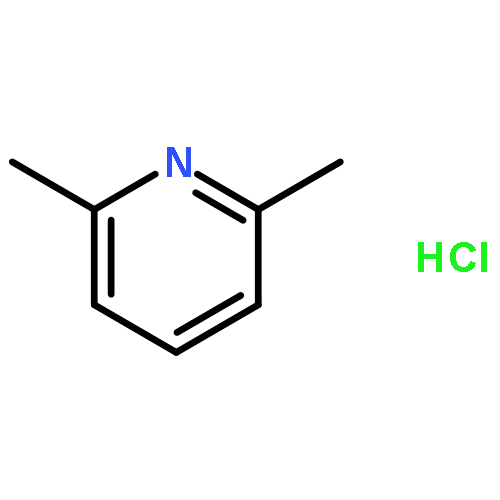
Tridentate PSiP pincer ligands featuring two phosphine donors and an anionic Si donor have attracted considerable attention in recent years. Here, we report the synthesis of the η3-cyclooctenyl complex, (PhPSiP)Ni(η3-cyclooctenyl) (1; PhPSiP = Si(Me)(2-PPh2–C6H4)2) through the reaction of Ni(COD)2 with PhPSiHP (PhPSiHP = HSi(Me)(2-PPh2–C6H4)2). We propose, that as a result of β-hydride elimination of 1,3-COD, 1 can act as a synthetic equivalent for (PhPSiP)NiH. The reaction of 1 with a variety of different reagents including another equivalent of PhPSiHP to form (PhPSiP)2Ni (2), 1,3-COD and H2, PPh3 to form the Ni(0) species (PhPSiHP)Ni(PPh3) (3) and 1,3-COD and 2,6-lutidine·HCl to generate (PhPSiP)NiCl (4), 1,3-COD and H2 are in agreement with this hypothesis. In addition, in the reaction of 1 with BH3·THF, (PhPSiP)Ni(κ2-BH4) (5) was observed but could not be isolated. This reaction presumably proceeds via (PhPSiP)NiH. This is supported by the observation that the reaction of (CyPSiP)NiH (CyPSiP = Si(Me)(2-PCy2-C6H4)2) with BH3·THF formed (CyPSiP)Ni(κ2-BH4) (6). Catalytic reactions such as alkene isomerization and CO2 reduction using 1 as precatalyst are also consistent with a nickel hydride being accessible. Compounds 1, 2 and 6 were characterized by X-ray crystallography.The synthesis and reactivity of the η3-cyclooctenyl complex, (PhPSiP)Ni(η3-cyclooctenyl)(PhPSiP = Si(Me)(2-PPh2–C6H4)2), which can act as a mask for a Ni hydride, is reported.
![Image for unlabelled figure]()